|
Drosophila cdi4 is a p21/p27/p57-like cyclin-dependent
kinase inhibitor with specificity for cyclin E complexes.
Please see notes
on this paper.
Russell L. Finley Jr 1.,
Barak Cohen 2, and Roger Brent 2
1 Center for Molecular Medicine and
Genetics
Wayne State University School of
Medicine
540 East Canfield Ave.
Detroit, Michigan 48201
USA
2 Department of Molecular Biology
Massachusetts General Hospital
50 Blossom Street
Boston, Massachusetts 02114
and Department of Genetics
Harvard Medical School
Boston, Massachusetts 02115
USA
Summary
The eukaryotic cell cycle is controlled by a network
of interacting regulatory proteins. We used an interaction mating two-hybrid
assay to identify connections within the cell cycle regulatory network
in Drosophila. We tested interactions between Drosophila
cyclins and a panel of hundreds of previously identified proteins. One
of the connections we identified was the interaction between cyclin E and
a novel Drosophila protein, Cdi4. Because Cdi4 was originally identified
by its ability to interact with a Drosophila cyclin-dependent kinase, the
finding that it interacts with cyclin E strengthened the notion that it
functions in cell cycle regulation. We show that Cdi4 can inhibit cyclin
E function both in a yeast assay and in vitro. In light of these results,
our sequence analysis revealed that Cdi4 is a unique member of the p21/p27/p57
family of Cdk inhibitors. Our results demonstrate that interaction mating
assays using large informative panels of proteins can aid the analysis
of regulatory networks by generating and constraining hypotheses that guide
further work.
Introduction
Biological processes are controlled by networks
of regulatory proteins. The function of individual members of these networks
is often not clear, and new members are often being identified more rapidly
than the functions of known members are determined. The network of proteins
that controls cell division in metazoans illustrates this point. Cell division
is controlled by numerous interacting cell cycle regulatory proteins. These
proteins include cyclin-dependent kinases (Cdks) and cyclins (Hunter and
Pines, 1994; Lees, 1995; Nigg, 1995; Nurse, 1994), and a number of Cdk
interacting proteins (Cdis), which modify Cdk activity (Sherr and Roberts,
1995), or which have uncharacterized functions (Finley et al., 1996).
In Drosophila, the G1 to S transition is
controlled in several tissues by developmental regulation of Cdks and cyclins.
For example, cyclin E expression is required for ectodermal and midgut
cells in the embryo to enter S phase (Duronio and O'Farrell, 1995; Knoblich
et al., 1994; Richardson et al., 1993; Sauer et al., 1995). In the developing
eye imaginal disc, cyclin E is expressed in clusters of cells about to
enter S phase posterior to the morphogenetic furrow. Cyclin D is also expressed
in the eye imaginal disc, but in a stripe at the anterior edge of the morphogenetic
furrow that preceeds but does not overlap cyclin E expression (Finley et
al., 1996; Richardson et al., 1995; Richardson et al., 1993). This pattern
is consistent with a model in which cyclin D stimulates the transition
from G1 arrest into G1 progression in response to external signals such
as developmental cues, and cyclin E then drives cells into S phase. Contrary
to this model, expression of cyclin D or cyclin E in some cells in the
developing eye disc is not sufficient to drive them out of G1 (Finley et
al., 1996; Richardson et al., 1995). These results have suggested that
there are other levels of developmental control over cyclin protein function.
However, few proteins that modulate Cdk/cyclin activity in Drosophila have
been identified.
Yeast two-hybrid systems have proven especially
useful in the identification and analysis of cell cycle regulatory proteins
(Durfee et al., 1993; Fields and Song, 1989; Finley and Brent, 1994; Gyuris
et al., 1993; Hannon et al., 1993; Harper et al., 1993). Drosophila
cyclin D and cyclin J, for example, were originally identified in a yeast
two-hybrid interactor hunt (Finley et al., 1996). Conventional yeast two-hybrid
methods, however, have not been effective to characterize cell cycle regulators
and other proteins that are strong transcription activators. For example,
Drosophila cyclin E is an unsatisfactory bait in such screens because
it activates transcription when brought to DNA. Moreover, high level constitutive
expression of cyclin E is toxic to yeast. Here we circumvented these problems
with a scaled-up version of a modified two-hybrid approach, interaction
mating (Finley and Brent, 1994), to identify proteins that might affect
cyclin E activity. In this technique, test proteins fused to a transcription
activation domain are conditionally expressed in one yeast strain. This
strain is mated to a large number of different strains containing different
LexA fusion baits, and reporter activation is assayed in the exconjugants.
This approach enabled us to quickly screen a panel of hundreds of previously
characterized bait proteins for interactions with cyclin E and other Drosophila
cyclins.
Here we show that Drosophila cyclin E interacts
specifically with the Drosophila Cdk interactor, Cdi4. We show that Cdi4
is a substrate for Cdk/cyclin E but not Cdk/cyclin D complexes in vitro,
and that Cdi4 inhibits Cdk/cyclin kinase activity. These results prompted
careful sequence analysis, which indicated that Cdi4 belongs to the p21/p27/p57
class of cell cycle inhibitors. These and other results demonstrate the
power of interaction mating to spark the generation of hypotheses about
gene function. This ability to generate hypotheses will be particularly
important in the analysis of the genomes of human and other organisms that
lack manipulative genetics.
Results
Interaction mating reveals specific association
of cyclin E with Cdi4
We collected 550 different bait plasmids from our
lab and from other investigators. These plasmids express fusions of LexA
to a variety of well or partially characterized proteins from Drosophila,
yeast, mammals, and other organisms. We introduced the bait plasmids into
a strain containing a sensitive LexAop-lacZ reporter (Colas et al.,
1996; Estojak et al., 1995) to construct a panel of bait strains. The panel
was arrayed in a 96-well pattern on yeast plates and then replica-mated
with a strain expressing a test protein such as Drosophila cyclin E fused
to a transcription activation domain. We scored interaction by blue color
after replica plating the exconjugants onto X-Gal medium. The technique
is illustrated in Fig. 1 (Experimental Procedures).
Figure 1 (full
size)
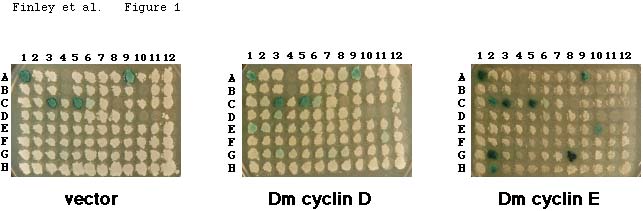
Figure 1 legend
We tested several Drosophila cyclins against
this panel. Fig. 1 shows a typical result, in which strains containing
either the vector alone, or expressing activation domain fusions to Drosophila
cyclin D or cyclin E, were mated with a set of panel members (128 different
baits). Some panel members contain baits that activate transcription on
their own (e.g., Fig. 1, position A1); we thus scored an interaction only
when we observed blue color that depended on the cyclin. Fig. 1, for example,
shows that the baits at positions G2, G8, and K12 interacted specifically
with cyclin E. The baits at positions G2, G8, and K12 are Drosophila
Rux, human p21CIP1/WAF1/Sdi1, and Drosophila Cdi4,
respectively. Fig. 1 also shows that cyclin E interacted specifically with
the baits at positions C2, E10, and H2. These are human p130, human p107,
and the C-terminus of yeast Ste7, respectively. These baits did not interact
with cyclin D (Fig. 1) or with cyclin C or cyclin J (Finley et al., 1996)
(not shown).
In addition to the interactions with the Drosophila
Cdks already described (Finley et al., 1996), the panel revealed several
interactions that were predictable and some that were unexpected (Table
1). For example, Cyclin D interacted strongly with human Cdk6, a partner
of human D cyclins (Meyerson and Harlow, 1994), and Cyclin E interacted
with human p21CIP1/WAF1, which inhibits Cdk/cyclin
activity (el-Deiry et al., 1993; Harper et al., 1993). Cyclin E also interacted
with two Rb-related proteins, p130, and p107, that are known to interact
with cyclins (Ewen et al., 1992; Hannon et al., 1993; Lees et al., 1992).
By contrast, Drosophila cyclin C (Lahue et al., 1991; Leopold and
O'Farrell, 1991) did not interact with any panel members (data not shown),
while Drosophila cyclin J interacted only with a Drosophila
Cdk (DmCdc2c) and yeast Cdc28, confirming previous work (Finley et al.,
1996).
Cyclin E also interacted unexpectedly with four
proteins not previously known to be cyclin interactors: HTLV-1 Tax protein,
a trasncription factor (Seiki et al., 1985); the C-terminus of the yeast
Ste7 protein, a protein kinase involved in signal transduction (Teague
et al., 1986); and two novel Drosophila proteins, Rux and Cdi4 (see
below). These findings suggested that these four proteins may play a role
in cell cycle regulation by directly interacting with cyclins, or alternatively
that their function may be directly modulated by cyclins. Here we explored
the possible significance to cell cycle regulation of the cyclin E interaction
with Cdi4. Cdi4 was already a suspected cell cycle regulator because it
was isolated in a hunt for proteins that contact one or more Drosophila
Cdks (Finley et al., 1996).
We confirmed interactions of cyclin E with Cdi4
by mating yeast that expressed activation domain-tagged Cdi4 with the bait
panel. Cdi4 interacted with both splice variant forms of Drosophila
cyclin E, type I and type II (Richardson et al., 1993), and with human
cyclin E, which is 41% identical to Drosophila cyclin E (data not
shown). Cdi4 also interacted with a cyclin E derivative that contains the
cyclin box (residues 193-517), a region of cyclins that contacts Cdks (Draetta,
1990; Lees and Harlow, 1993)
Cdi4 inhibits cyclin E activity in yeast
We used a yeast assay to test whether Cdi4 can
regulate cyclin E activity. We tested whether Cdi4 could prevent the toxic
effect of human cyclin E in yeast. In yeast, high level expression of cyclin
E inhibits growth (see below), perhaps because it causes inappropriate
activation of the yeast Cdk, Cdc28. Table 2 shows
this effect. Compared with a control plasmid, human cyclin E causes a 10-fold
reduction in transformation efficiency. Table 2 also shows that co-transformation
of the cyclin E plasmid along with a plasmid expressing Cdi4 from the yeast
GAL1 promoter increased the transformation efficiency over 10-fold
(Table 2). This rescue depended on Cdi4 expression,
as it was only observed when transformants were plated on galactose medium
(not shown). These results suggest that the interaction of Cdi4 with cyclin
E inhibited Cdk/cyclin E activity.
Cdi4 is a substrate for Cdk/cyclin complexes
in vitro and inhibits Cdk/cyclin kinase activity
We further tested interaction in vitro by
determining whether Cdi4 was phosphorylated by complexes containing cyclin
E. Fig. 2a shows that Cdi4 (and Rux) fusion proteins were phosphorylated
by human Cdk2 and cyclin A (Cdk2/cyclin A), Cdk2 and cyclin E (Cdk2/cyclin
E), but not by Cdk4 and cyclin D1 complexes (Cdk4/cyclin D), further suggesting
that these interactions were functional.
Cdi4 inhibited the kinase activity of the Cdk2/cyclin
E or Cdk2/cyclin A combinations (Fig. 2b, 2c). MBP-Cdi4 inhibited both
Cdk/cyclin complexes by well over 50% (Fig 2b and 2c, lanes 4) when present
at less than 2-fold molar excess over the histone H1 substrate (although
we do not know what proportion of the bacterially-expressed fusion protein
is active). Inhibition of kinase activity increased by over 10-fold when
the amount of MBP-Cdi4 in the reaction was doubled (compare lanes 3 and
4 in Fig. 2b and 2c), suggesting that inhibition by Cdi4 may be cooperative.
Cdi4 did not inhibit kinase activity of the Cdk4/cyclin D1 lysates (Fig.
2d), consistent with the two hybrid and in vitro results showing
that Cdi4 interacts only weakly or not at all with D type cyclins.
Figure 2 (full
size)

Figure 2 legend
Cdi4 is a member of p21/p27/p57 family of cyclin-dependent
kinase inhibitors.
Having shown that Cdi4 inhibited Cdk/cyclin activity,
and that it can inhibit cyclin E toxicity in yeast, we compared its sequence
carefully to that of known Cdk inhibitors, or Ckis (Sherr and Roberts,
1995). We found that Cdi4 has sequence similarity with the p21/p27/p57
class of Ckis (Fig. 3). Members of this class include mammalian p21CIP1/WAF1/Sdi1
(here called p21) (el-Deiry et al., 1993; Harper et al., 1993; Noda et
al., 1994; Xiong et al., 1993), p27KIP1 (here called
p27)(Polyak et al., 1994), and p57KIP2 (here called
p57) (Lee et al., 1995; Matsuoka et al., 1995), a Xenopus p27 (Su et al.,
1995), and a C.elegans Cki with similarity to p21. Most of the similarity
between these proteins lies in their amino terminal 90 residues (see Fig.
3b). In human p21, this region interacts with Cdk/cyclin complexes and
inhibits their kinase activity (Chen et al., 1995; Goubin and Ducommun,
1995; Luo et al., 1995; Nakanishi et al., 1995). This region of Cdi4 contains
10 residues found in all p21/p27/p57 family members, and an additional
3 amino acids found in at least 6 of these Ckis. The middle of Cdi4 (from
92 to 161) has no significant similarity with other Ckis, but the carboxy
terminus has separate regions that resemble p21 and p57. From residues
162-185, Cdi4 contains 7 identical and 6 similar amino acids found in a
region of p21s but not other Ckis; in human p21, this portion of the protein
interacts with PCNA (Chen et al., 1995; Luo et al., 1995). From residues
186 to the C-terminus, Cdi4 contains a region rich in prolines and alanines,
which includes a PAPA repeat, similar to a region found in p57s but not
other Ckis (Fig. 3d) (Lee et al., 1995; Matsuoka et al., 1995). Combined,
these results indicate that Cdi4 is a Cki that shares characteristics with
both the p21 and p57 proteins.
Figure 3 (full
size)

Figure 3 legend
Discussion
Two-hybrid systems have been enormously useful
for identification of new genes, for example those involved in cell cycle
regulation (Allen et al., 1995; Mendelsohn and Brent, 1994; Phizicky and
Fields, 1995). Extensions of these systems, such as interaction mating,
have also begun to find use for charting genetic pathways and assessing
gene function (Finley et al., 1994). Here we applied these methods on a
larger scale to study the protein network that regulates the cell cycle
in response to developmental cues in Drosophila. We began with cyclins,
which have often been refractory to traditional two-hybrid methods because
they activate transcription as baits and sometimes are toxic in yeast.
In these experiments, we expressed Drosophila
cyclins conditionally and tested their ability to interact with a large
panel of previously identified bait proteins. The baits in this panel came
from our lab and from hundreds of other labs using the yeast two-hybrid
system. The panel comprises a set enriched in proteins of interest to contemporary
biologists; most of which are known members of one or more regulatory networks.
Our experiments revealed four previously unsuspected interactions between
Drosophila cyclin E and members of the panel. Although they were
above the detection threshold, and conceivably significant, we did not
pursue two of these: HTLV-1 tax and S. cerevisiae Ste7. A third,
Drosophila Rux, is a negative regulator of cell cycle progression required
for proper eye development (Thomas et al., 1994). Our results suggest that
at least one way Rux might function is by direct interaction with cyclins
(Thomas et al., 1997). Here we explored another interaction that seemed
to make sense for cell cycle regulation, between cyclin E and Drosophila
Cdi4.
We have shown here that Cdi4, which was isolated
because it interacts with a Drosophila Cdk, DmCdc2c, interacts with
cyclin E and inhibits its activity. This finding prompted us to carefully
compare the Cdi4 sequence to that of other inhibitors of Cdk/cyclin activity
(Ckis). We found that, although Cdi4 has low overall similarity to individual
Ckis, it contains sequence motifs conserved in the amino termini of the
p21/p27/p57 class of Ckis, a portion of these proteins that is thought
to contact Cdks and cyclins (Chen et al., 1995; Lin et al., 1996; Luo et
al., 1995; Nakanishi et al., 1995). It also contains separate regions at
its C-terminus which resemble p21s and p57s, respectively. The C-terminal
parts of these proteins are not conserved and may have different functions.
The C-terminal half of p21, for example, interacts with PCNA and inhibits
PCNA-dependent DNA replication, but the C-terminus of p57 does not (Chen
et al., 1995; Luo et al., 1995). Whether or not Cdi4 interacts with PCNA,
our results clearly indicate that Cdi4 is a p21 and p57-like Drosophila
Cki that could inhibit cell cycle progression by blocking kinase activity.
p21/p27/p57 Ckis have a higher affinity for Cdk/cyclin
complexes than for monomeric Cdks (Sherr and Roberts, 1995). This is consistent
with the idea that these Ckis make contacts both with Cdks and with cyclins
(Lin et al., 1996). Our results are consistent with this idea: they suggest
that Cdi4 makes individual binary contacts, with an affinity detectable
with our two hybrid system, both with a Drosophila Cdk and cyclin
E. Moreover, our results show that Cdi4 specifically inhibits Cdk/cyclin
A or Cdk/cyclin E complexes, and not of Cdks complexed with cyclin C, cyclin
J, or cyclin D, suggesting that the different affinities of Cdi4 for the
different cyclins are decisive in determining which complexes it inhibits.
The affinity of Cdi4 for cyclin E, which is required for the G1 to S phase
transition (Knoblich et al., 1994), suggests that Cdi4 may inhibit entry
into S, perhaps in response to developmental signals. The spurious rounds
of S phase observed in dacapo Drosophila mutants, and the
recent finding that these mutants bear lesions in the Cdi4 coding region,
are consistent with this idea (I. Hariharan and C. Lehner, personal communication).
Our results clearly demonstrate that interaction
mating against panels of characterized proteins can provide insights into
the function of both interacting partners. These insights come from the
ability of this technique to suggest easily testable hypotheses about the
interacting partners. The power of the technique grows as the panel grows:
currently, it consists of more than 700 proteins, each of which has been
at least somewhat characterized. The emergence of this technique is timely,
since the need to assign function to genes, particularly those with no
sequence similarity to known genes, and those from organisms without well
developed genetics, is great. Our results with Cdi4 and Rux demonstrate
that interaction mating followed by ad hoc experiments to verify the conclusions
may provide a quick route to this task.
Methods
Yeast strains and manipulations
Saccharomyces cerevisiae yeast strains used were RFY206 (MATa
his3Æ200 leu2-3 lys2Æ201 ura3-52 trp1Æ::hisG) (Finley
and Brent, 1994), EGY48 (MATa
his3 ura3 trp1 LYS2 leu2::3Lexop-LEU2) (Estojak et al., 1995), EGY40
(Mata his3 ura3
trp1 leu2 LYS2) (E. Golemis and R. Brent, unpublished), and 3c1Ax
(MATa bar1 trp1 leu2 ura3 ade1 cyh2 his2 Æcln1 Æcln2 Æcln3
[pLEU2-CYH2-CLN3]) (provided by J. Roberts and F. Cross). Yeast
were grown using standard microbiological techniques and media (Ausubel
et al., 1987-1997; Guthrie and Fink, 1991). Media designations are as follows:
YPD is YP (yeast extract plus peptone) medium with 2% glucose. Minimal
dropout media are designated by the component that is left out (e.g. -ura
-his -trp -leu medium lacks uracil, histidine, tryptophan, and leucine).
Minimal media contained either 2% glucose (Glu) or 2% galactose plus 1%
raffinose (Gal). X-Gal minimal dropout plates contained X-Gal and phosphate
buffer at pH 7.0. DNA was introduced into yeast by LiOAc-mediated transformation
as described (Gietz et al., 1992).
Plasmids
Most bait plasmids are derived from pLEX(202+PL) (Ruden et al., 1991)
or pEG202 (Estojak et al., 1995), which both contain the HIS3 gene,
2µm origin of replication, and the ADH1 promoter driving expression
of fusion proteins with amino acids 1 to 202 of LexA at their amino termini.
A small number of the bait plasmids are derived from either pNLex (provided
by B. Vogelstien) which is derived from pLEX(202+PL) but encodes LexA with
a nuclear localization signal at its C-terminus prior to the fusion protein,
or pNLexA (provided by I. York) which encode fusions with LexA at their
C-terminal rather than N-terminal end. Bait plasmids that expressed variants
of Drosophila cyclin E were derived from pGILDA (D. Shaywitz and
C. Kaiser, personal communication) in which the LexA fusion protein is
expressed from the GAL1 promoter instead of the ADH1 promoter,
allowing transient expression of toxic proteins. Bait plasmids for which
interactions are reported here are as follows: pRF202-Cdi4 is pEG202 cut
with EcoRI and XhoI with an inserted a 876 bp MunI-XhoI fragment of the
original Cdi4 cDNA (Finley et al., 1996) generated by polymerase chain
reaction (PCR) with the 5’ MunI site introduced in the 5’ primer (CAATTGCAAGGCAGCCCGGCGGTGAGTCG)
and the 3’ XhoI site from the original cDNA downstream of the stop codon;
this plasmid encodes the entire 255 amino acid Cdi4 protein shown in Fig.
4a with an additional glutamine (Q) and leucine (L) encoded by the MunI
site; pKZ202-Rux is pEG202 cut with BamHI, filled-in with Klenow, then
cut with XhoI, with an inserted 1.22 kb fragment encoding amino acid 2
to the C-terminus of Rux; pRF202-DmcycE(193-517) is pEG202 cut with EcoRI
and XhoI with an inserted 980 bp PCR-generated EcoRI-XhoI fragment encoding
amino acids 193-517 of Drosophila cyclin E type I (numbering system
of Richardson et al. (Richardson et al., 1993)). Plasmids encoding LexA
fusions to human Cdk6 and human p21CIP1/WAF1/SDI1
have been described (Reymond and Brent, 1995). Other bait plasmids encoded
human p130 or p107 (provided by C. Sardet and R. Weinberg), amino acids
322-1139 of human p130 (provided by A. Bannister and T. Kouzarides), HTLV-1
Tax (provided by K. Clemens), and the C-terminal 344 amino acids of S.
cerevisiae Ste7 (provided by B. Satterberg and E. Elion). The 2 µm
URA3 lacZ reporter pSH18-34 containing four LexA operators upstream
of a GAL1-lacZ fusion, pSH18-34, has been described. Derivatives
of pJG4-5 (Gyuris et al., 1993) that expressed activation domain-tagged
fusions to Drosophila cyclin D (p4-5-Cdi3), cyclin J (p4-5-Cdi5),
and Cdi4 (p4-5-Cdi4) were originally isolated in two-hybrid hunts for Drosophila
Cdk interactors (Finley et al., 1996). The pJG4-5 derivative that expressed
activation domain-tagged Drosophila cyclin E (p4-5-Cdi7ÆN)
encodes from amino acid 38 to the C-terminus of Drosophila cyclin
E type II (numbering system of Richardson et al. (Richardson et al., 1993).
p4-5-Rux was made by inserting the EcoRI fragment from pKZLex-Rux encoding
amino acids 2 to the C-terminus of Rux into the EcoRI site of pJG4-5. pHC21
(H. Chertkov, J. Gyuris, R.Brent, unpublished) is a 2µm URA3
plasmid containing an ADH1 promoter and terminator expression cassette.
pHC21-HsCycE (H. Chertkov, J. Gyuris, R.Brent, unpublished) expresses full-length
human cyclin E from the ADH1 promoter. pRF4-6o is a 2µm TRP1
plasmid made by inserting an EcoRI-XhoI ended 36 bp oligonucleotide containing
multiple unique restriction sites into pJG4-6 (J. Gyuris and R. Brent,
unpublished) cut with EcoRI and XhoI; the unique restriction sites reside
between the yeast GAL1 promoter and ADH1 terminator, just downstream
of an ATG and coding region for the 7 amino acid heamaglutinin epitope
tag (HA). Coding regions for Rux, Cdi4, or Cdi11 (Finley et al., 1996)
were inserted into the unique sites of pRF4-6o to allow galactose-dependent
expression of these proteins with an HA tag at their amino termini. Vectors
for expressing fusions of maltose binding protein (MBP) to Rux or Cdi4
were made by inserting the EcoRI fragment from pKZ-Rux or a MunI-XhoI PCR
fragment of Cdi4 (see above) into the EcoRI cut or EcoRI/XhoI cut pMAL-c2
(New England Biolabs).
Panel of baits
We collected 550 bait plasmids that expressed LexA
fusion proteins. About 150 of the plasmids were made in our own labs and
the remainder were kindly donated by other labs using the two-hybrid system.
A complete list of the bait plasmids in the panel is available on request
or can be obtained at http://www.xanadu.mgh.harvard.edu/. Bait strains
were created by transforming yeast strain RFY206 with the lacZ reporter
pSH18-34 and individual bait plasmids. Bait strains were selected and maintained
on glucose minimal medium lacking uracil and histidine (-u-h Glu), and
were stored frozen by resuspending fresh cultures in 1:1 -u-h Glucose medium:glycerol
solution (Finley and Brent, 1996). The 550 bait strains were arrayed in
a 96-well configuration on 150 mm -u-h glucose plates (~96 bait strains/plate).
Bait strains were transferred to new plates or to liquid cultures from
saturated 4 ml -u-h Glu cultures in 96-well cluster tubes using a 96 prong
device.
Interaction mating
Interaction mating assays were performed essentially
as previously described (Finley and Brent, 1994; Finley and Brent, 1995;
Finley and Brent, 1996). The entire panel of 550 bait strains were arrayed
on six 150 mm plates with up to 96 different strains on each plates. "Prey"
strains were EGY48 containing pJG4-5 or derivatives of pJG4-5 that expressed
individual cyclins or other proteins with a transcription activation domain
fused to their amino terminal end. Prey strains were grown in -w Glu liquid
medium to saturation and transferred to 150 mm plates in a 96-well configuration
using a 96 prong device. Bait and prey strain plates were grown for two
days at 30oC and then replica mated by pressing both
plates to the same replica velvet and lifting the impression with a single
150 mm YPD plates. The YPD plates were incubated at 30oC
for one day then replica plated to two X-Gal indicator that lack uracil,
tryptophan, and histidine so that only diploids could grow, and that contain
either 2% glucose (Glu) or 2% galactose (plus 1% raffinose to aid growth)
to induce expression of the activation domain-tagged protein. An interaction
was scored when a diploid strain turned bluer on the Gal X-Gal plate than
on the Glu X-Gal, and that was also bluer in the presence of an activation
domain-tagged protein than with the vector alone.
Yeast assays
In separate experiments yeast strain EGY40 or 3c1Ax
were co-transformed using the lithium acetate method (Gietz et al., 1992)
with 0.5 µg each of a Cen/Ars URA3 plasmid and a TRP1
plasmid and plated at various dilutions on glucose medium lacking uracil
and tryptophan. The URA3 plasmid was either pHC21 (H. Chertkov and
R.B., unpublished), or pHC21-HsCycE that expressed human cyclin E from
the yeast ADH1 promoter. The TRP1 plasmid was pRF4-6o, pRF4-6-Rux,
pRF4-6-Cdi4, or pRF4-6-Cdi11. Transformant colonies were counted after
2 days.
Kinase assays
in vitro kinase assays were performed using
lysates from baculovirus infected Sf9 cells essentially as described (Kato
et al., 1993). Sf9 cells were co-infected with recombinant baculoviruses
expressing human His6-tagged cyclin E and HA-tagged Cdk2 (provided by D.
Morgan), HA-tagged Cdk2 and GST-tagged cyclin A (provided by H. Piwinica-Wroms),
or mouse Cdk4 and cyclin D1 (provided by C. Sherr), or no Cdk and cyclin
(mock) at an MOI of 10 for each virus. Cells were grown at 27oC
in TMN-FH plus 10% fetal bovine serum (FBS) in 100 ml spinner flasks for
40 hours, then harvested by centrifugation, washed once in PBS, and resuspended
in cold 500 µl lysis buffer (50 mM HEPES pH7.5, 10 mM MgCl2,
1 mM DTT, 10 mM ß-glycerophosphate, 0.1 mM each PMSF, NaF, Na orthovanadate,
and 5 µg/ml each aprotinin and leupeptin) . The lysates were incubated
on ice for 1 hour, then clear by centrifugation at 10,000 x g for 20 min.
Aliquots were frozen at -80oC. Kinase assays were
performed in 20 µl of lysis buffer using 2 µl of lysate diluted
100-fold (Cdk2/cyclin E and Cdk4/cyclin D1) or 50-fold (Cdk2/cyclin A)
in the presence of 25 µM ATP and 2.5 µCi 32P-g-ATP
(3000 Ci/mmol), and either 0.2 µg histone H1, 0.3 µg bacterially
expressed and affinity purified GST-Rb (provided by A. Reymond), or bacterially
expressed and affinity purified fusions of maltose binding protein (MBP)
to Cdi4 or Rux. Kinase assays were incubated at 25oC
for 20 min. , stopped by adding 10 µl Laemmli sample buffer, and
analyzed on 10 % PAGE-SDS gels.
Acknowledgments
We thank I. Hariharin, C. Lehner, and H. Richardson
for communication of unpublished data. We thank H. Piwinca-Worms for GST-cyclin
A baculovirus plasmid, D. Morgan for 6His-cyclin A and HA-Cdk2 baculovirus
plasmids, and C. Sherr for Cdk4 and cyclin D1 baculovirus plasmids. We
also thank Pierre Colas and Andy Mendelsohn for comments on the manuscript.
References
Ausubel, F. M., Brent, R., Kingston, R. E., Morre, D., Seidman,
J. G., and Struhl, K. (1987-1997). Current protocols in molecular biology
(New York: Greene and Wiley-interscience).
Chen, J., Jackson, P. K., Kirschner, M. W., and Dutta, A. (1995).
Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA.
Nature 374, 386-388.
Colas, P., Cohen, B., Jessen, T., Grishina, I., McCoy, J., and Brent,
R. (1996). Genetic selection of peptide aptamers that recognize and inhibit
cyclin- dependent kinase 2. Nature 380, 548-550.
Draetta, G. (1990). Cell cycle control in eukaryotes: molecular mechanisms
of cdc2 activation. Trends Biochem. Sci. 15, 378-382.
Durfee, T., Becherer, K., Chen, P. L., Yeh, S. H., Yang, Y., Kilburn,
A. E., Lee, W. H., and Elledge, S. J. (1993). The retinoblastoma protein
associates with the protein phosphatase type 1 catalytic subunit. Genes
Dev 7, 555-569.
Duronio, R. J., and O'Farrell, P. H. (1995). Developmental control
of the G1 to S transition in Drosophila: cyclin Eis a limiting downstream
target of E2F. Genes Dev 9, 1456-1468.
el-Deiry, W. S., Tokino, T., Velculescu, V. E., Levy, D. B., Parsons,
R., Trent, J. M., Lin, D., Mercer, W. E., Kinzler, K. W., and Vogelstein,
B. (1993). WAF1, a potential mediator of p53 tumor suppression. Cell 75,
817-25.
Estojak, J., Brent, R., and Golemis, E. A. (1995). Correlation of
two-hybrid affinity data with in vitro measurements. Mol Cell Biol 15,
5820-5829.
Ewen, M. E., Faha, B., Harlow, E., and Livingston, D. M. (1992).
Interaction of p107 with cyclin A independent of complex formation with
viral oncoproteins. Science 255, 85-7.
Fields, S., and Song, O. (1989). A novel genetic system to detect
protein-protein interactions. Nature 340, 245-246.
Finley, R. L., Jr., and Brent, R. (1994). Interaction mating reveals
binary and ternary connections between Drosophila cell cycle regulators.
Proc Natl Acad Sci U S A 91, 12980-12984.
Finley, R. L., Jr., and Brent, R. (1995). Interaction trap cloning
with yeast. In DNA Cloning, Expression Systems: A Practical Approach, B.
D. Hames and D. M. Glover, eds. (Oxford: Oxford University Press), pp.
169-203.
Finley, R. L., Jr., and Brent, R. (1996). Two-hybrid analysis of
genetic regulatory networks. In The yeast two-hybrid system, P. L. Bartel
and S. Fields, eds. (Oxford: Oxford University Press).
Finley, R. L., Jr., Thomas, B. J., Zipursky, S. L., and Brent, R.
(1996). Isolation of Drosophila cyclin D, a protein expressed in the morphogenetic
furrow before entry into S phase. Proc. Natl. Acad. Sci. USA 93,
3011-3015.
Gietz, D., St. Jean, A., Woods, R. A., and Schiestl, R. H. (1992).
Improved method for high efficiency transformation of intact yeast. Nuc.
Acids Res. 20, 1425.
Goubin, F., and Ducommun, B. (1995). Identification of binding domains
on the p21Cip1 cyclin-dependent kinase inhibitor. Oncogene 10, 2281-2287.
Guthrie, C., and Fink, G. R. (1991). Guide to yeast genetics and
molecular biology. In Methods in enzymology (Boston: Academic Press, Inc.).
Gyuris, J., Golemis, E., Chertkov, H., and Brent, R. (1993). Cdi1,
a human G1 and S phase protein phosphatase that associates with Cdk2. Cell
75, 791-803.
Hannon, G. J., Demetrick, D., and Beach, D. (1993). Isolation of
the rb-related p130 through its interaction with cdk2 and cyclins. Genes
& Development 7, 2378-2391.
Harper, J. W., Adami, G. R., Wei, N., Keyomarsi, K., and Elledge,
S. J. (1993). The p21 cdk-interacting protein cip1 is a potent inhibitor
of g1 cyclin-dependent kinases. Cell 75, 805-816.
Hunter, T., and Pines, J. (1994). Cyclins and Cancer II:
Cyclin D and CDK inhibitors come of age. Cell 79, 573-582.
Kato, J., Matsushime, H., Hiebert, S. H., Ewen, M. E., and Sherr,
C. J. (1993). Direct binding of cyclin D to the retinoblastoma gene product
(pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes
and Development 7, 331-342.
Knoblich, J. A., Sauer, K., Jones, L., Richardson, H., Saint, R.,
and Lehner, C. F. (1994). Cyclin E controls s phase progression and its
down-regulation during drosophila embryogenesis is required for the arrest
of cell proliferation. Cell 77, 107-120.
Lahue, E. E., Smith, A. V., and Orr-Weaver, T. L. (1991). A novel
cyclin gene from Drosophila complements CLN function in yeast. Genes Dev
5, 2166-75.
Lee, M. H., Reynisdottir, I., and Massague, J. (1995). Cloning of
p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure
and tissue distribution. Genes Dev 9, 639-649.
Lees, E. (1995). Cyclin dependent kinase regulation. Curr. Op. in
Cell Biol. 7, 773-780.
Lees, E., Faha, B., Dulic, V., Reed, S. I., and Harlow, E. (1992).
Cyclin E/cdk2 and cyclin A/cdk2 kinases associate with p107 and E2F in
a temporally distinct manner. Genes Dev 6, 1874-85.
Lees, E. M., and Harlow, E. (1993). Sequences within the conserved
cyclin box of cyclin A are sufficient for binding to and activation of
cdc2 kinase. Mol. Cell. Biol. 13, 1194-1201.
Leopold, P., and O'Farrell, P. H. (1991). An evolutionary conserved
cyclin homolog from Drosophila rescues yeast deficient in G1 cyclions.
Cell 66, 1207-1216.
Lin, J., Reichner, C., Wu, X., and Levine, A. J. (1996). Analysis
of wild-type and mutant p21WAF1 gene activities. Mol. Cell. Biol.
16, 1786-1793.
Luo, Y., Hurwitz, J., and Massague, J. (1995). Cell-cycle inhibition
by independent CDK and PCNA binding domains in p21Cip1. Nature 375,
159-161.
Matsuoka, S., Edwards, M. C., Bai, C., Parker, S., Zhang, P., Baldini,
A., Harper, J. W., and Elledge, S. J. (1995). p57KIP2, a structurally distinct
member of the p21CIP1 Cdk inhibitor family, is a candidate tumor suppressor
gene. Genes Dev 9, 650-662.
Mendelsohn, A. R., and Brent, R. (1994). Applications of interaction
traps/two-hybrid systems to biotechnology research. Curr Opin Biotechnol
5, 482-486.
Meyerson, M., and Harlow, E. (1994). Identification of G1 kinase
activity for cdk6, a novel cyclin D partner. Mol Cell Biol 14, 2077-86.
Nakanishi, M., Robetorye, R. S., Adami, G. R., Pereira-Smith, O.
M., and Smith, J. R. (1995). Identification of the active region of the
DNA synthesis inhibitory gene p21Sdi1/CIP1/WAF1. Embo J 14, 555-563.
Nakanishi, M., Robetorye, R. S., Pereira-Smith, O. M., and Smith,
J. R. (1995). The C-terminal region of p21SDI1/WAF1/CIP1 is involved in
proliferating cell nuclear antigen binding but does not appear to be required
for growth inhibition. J Biol Chem 270, 17060-17063.
Nigg, E. A. (1995). Cyclin-dependent protein kinases: key regulators
of the eukaryotic cell cycle. Bioessays 17, 471-480.
Noda, A., Ning, Y., Venable, S. F., Pereira-Smith, O. M., and Smith,
J. R. (1994). Cloning of senescent cell-derived inhibitors of DNA synthesis
using an expression screen. Exp Cell Res 211, 90-8.
Nurse, P. (1994). Ordering S phase and M phase in the cell cycle.
Cell 79, 547-550.
Phizicky, E. M., and Fields, S. (1995). Protein-protein interactions:
methods for detection and analysis. Microbiol Rev 59, 94-123.
Polyak, K., Kato, J. Y., Solomon, M. J., Sherr, C. J., Massague,
J., Roberts, J. M., and Koff, A. (1994). P27(kip1), a cyclin-cdk inhibitor,
links transforming growth factor-beta and contact inhibition to cell cycle
arrest. Genes & Development 8, 9-22.
Reymond, A., and Brent, R. (1995). p16 proteins from melanoma-prone
families are deficient in binding to Cdk4. Oncogene 11, 1173-1178.
Richardson, H., O'Keefe, L. V., Marty, T., and Saint, R. (1995).
Ectopic cyclin E expression induces premature entry into S phase and disrupts
pattern formation in the Drosophila eye imaginal disc. Development
121, 3371-3379.
Richardson, H. E., Okeefe, L. V., Reed, S. I., and Saint, R. (1993).
A drosophila G(1)-specific cyclin E homolog exhibits different modes of
expression during embryogenesis. Development 119, 673-690.
Ruden, D. M., Ma, J., Li, Y., Wood, K., and Ptashne, M. (1991). Generating
yeast transcriptional activators containing no yeast protein sequences.
Nature 350, 250-252.
Sauer, K., Knoblich, J. A., Richardson, H., and Lehner, C. F. (1995).
Distinct modes of cyclin E/cdc2c kinase regulation and S-phase control
in mitotic and endoreduplication cycles of Drosophila embryogenesis. Genes
Dev 9, 1327-1339.
Seiki, M., Inoue, J., Takeda, T., Hikikoshi, A., Sato, M., and Yoshida,
M. (1985). The p40x of human T-cell leukemia virus type I is a trans-acting
activator of viral gene transcription. Jpn J Cancer Res 76, 1127-31.
Sherr, C. J., and Roberts, J. M. (1995). Inhibitors of mammalian
G1 cyclin-dependent kinases. Genes & Dev. 9, 1149-1163.
Su, J. Y., Rempel, R. E., Erikson, E., and Maller, J. L. (1995).
Cloning and characterization of the Xenopus cyclin-dependent kinase inhibitor
p27XIC1. Proc Natl Acad Sci U S A 92, 10187-10191.
Teague, M. A., Chaleff, D. T., and Errede, B. (1986). Nucleotide
sequence of the yeast regulatory gene STE7 predicts a protein homologous
to protein kinases. Proc Natl Acad Sci U S A 83, 7371-5.
Thomas, B. J., Gunning, D. A., Cho, J., and Zipursky, L. (1994).
Cell cycle progression in the developing Drosophila eye: roughex encodes
a novel protein required for the establishment of G1. Cell 77, 1003-1014.
Thomas, B. J., Zavitz, K., Dong, X., Lane, M. E., Weigmann, K., Finley,
J., R.L., Brent, R., Lehner, C. F., and Zipursky, S. L. (1997). Roughex
downregulates G2 cyclin in G1. Genes & Develpopment 11, 1289-1298.
Xiong, Y., Hannon, G. J., Zhang, H., Casso, D., Kobayashi, R., and
Beach, D. (1993). P21 is a universal inhibitor of cyclin kinases. Nature
366, 701-704.
Table 1. Summary
of cyclin interactions with members of the panel of baits.
| Bait |
Cyc E
|
Cyc D
|
Vect.
|
| Hs Cdk6 |
-
|
+++
|
-
|
| Hs p21CIP1/WAF1/Sdi1 |
+++
|
-
|
-
|
| Hs p130 |
++
|
-
|
-
|
| Hs p107 |
++
|
-
|
-
|
| HTLV-1 tax |
++
|
-
|
-
|
| Sc Ste7 (C-terminus) |
++
|
-
|
-
|
| Dm Rux |
+++
|
-
|
-
|
| Dm Cdi4 |
+++
|
+
|
-
|
Interaction mating was performed as described in Experimental
Procedures and shown in Fig. 1. Level of interaction as determined by blue
color on X-Gal indicator plates: +++ indicates dark blue, ++ light blue,
+ very light blue, and - white. For baits that activate transcription on
there own, the amount of increase in blue color in the presence of the
cyclin relative to its absence is reported
.
Table 2. Rux and
Cdi4 overcome the toxic effect of Cyclin E overexpression in yeast.
| |
pHC21 |
pHC21-HsCycE |
| pRF4-6o |
152 ± 12 |
16 ± 4 |
| pRF4-6-Rux |
82 ± 5 |
174 ± 18 |
| pRF4-6-Cdi4 |
94 ± 2 |
150 ± 7 |
| pRF4-6-Cdi11 |
166 ± 13 |
20 ± 4 |
Numbers indicate transformants/0.1 µg of pHC21
or pRFHC21-HsCycE in double transformations of yeast strain EGY40 with
0.5 µg of pHC21 or pHC21-HsCycE and 0.2 µg of pRF4-6 or derivatives
expressing Rux, Cdi4, or Cdi11. Transformations were plated on selective
medium containing galactose. The average of two experiments are shown with
the difference between the two as the variance. Similar results were obtained
with yeast strain 3c1Ax (data not shown).
Figure Legends
Fig. 1. 96-well interaction mating to identify
cyclin interactors. The three panels show X-Gal indicator plates containing
mating exconjugants from matings of a yeast strain expressing Drosophila
cyclin E (Dm cyclin E), cyclin D (Dm cyclin D), or the vector alone, with
90 different bait strains. Interaction mating was performed as described
(see Methods; (Finley and Brent, 1994; Finley and Brent, 1996). Bait strains
containing the lacZ reporter gene and arrayed in a 96-well format
were mated by replica plating with strains that expressed the cyclins fused
to an activation domain. One 96-well panel plate (rows A to H) plus three
rows of a second 96-well panel plate (rows I,J,K) are shown. Cyclin E-dependent
activation of the lacZ reporter indicates that Dm cyclin E interacted
with baits at positions C2 (amino acids 322-1139 of human p130), E10 (human
p107), G2 (Drosophila Rux), G8 (human p21Waf1/CIP1/Sdi1), H2 (C-terminal
portion of yeast Ste7), and K12 (Drosophila Cdi4). The baits in
the strains at positions A1, A9, C3, C5, and K1 activated transcription
of the lacZ reporter on their own, i.e., even in the absence of
a cyclin. Dm cyclin D did not interact with any of these 90 baits at this
sensitivity. 6 positions lacked baits strains (A4, B4, B10, C8, D10, D11).
Back to Figure 1 place in text
Fig. 2. Cdi4 is an in vitro substrate of
Cdk/cyclins and inhibits kinase activity. a, Lysates from cells
infected with baculovirus expressing human Cdk2 and cyclin A (lanes 1-3),
human Cdk2 and cyclin E (lanes 4-6), mouse Cdk4 and cyclin D1 (lanes 9-12),
or not infected ("mock", lanes 7 and 8) were used to phosphorylate bacterially
expressed, affinity purified maltose binding protein (MBP) or MBP fused
to Cdi4, or GST-pRb. Lysates were diluted to achieve approximately equal
levels of phosphorylation of histone H1 or pRb (not shown). b, Cdk2/cyclin
E lysates were incubated with 0.2 µg of histone H1 (H1) and 0, 0.4,
0.8, 1.6, 3.2, 6.4 µg of MPB-Cdi4 (lanes 1-6, respectively), or 6.4
µg MBP (lane 7). Lane 8 contains 6.4 µg MBP-Cdi4 but no H1.
c, Cdk2/cyclin A lysates were incubated with 0.2 µg of histone
H1 (H1) and 0, 0.4, 0.8, 1.6, 3.2, or 6.4 µg of MPB-Cdi4 (lanes 1-6,
respectively), or 6.4 µg MBP (lane 7). Lane 8 contains 6.4 µg
MBP-Cdi4 but no H1. d, Cdk4/cyclin D1 lysates were incubated with
0.3 µg of GST-Rb (pRb) and 0, 0.4, 0.8, 1.6, 3.2 µg of MPB-Cdi4
(lanes 1-5, respectively). Back to Figure 2
place in text
Fig. 3. a, Predicted amino acid sequence
encoded by the Drosophila Cdi4 cDNA (Finley et al., 1996). b,
Alignment of first 88 residues of Cdi4 with the amino terminal portions
of mouse, human, and rat p21, mouse and human p27 and p57, Xenopus p27,
and a C.elegans p21-like protein. Shaded amino acids occur in at least
six of the 10 Ckis. Amino acids shown below Cdi4 are found in at least
9 of the 10 Ckis. c, Residues 162 to 185 of Cdi4 aligned with regions
in the C-terminal half of mouse and human p21. Shaded amino acids are identical
in all three proteins; boxed amino acids are conserved. d, Residues
186 to 213 of Cdi4 aligned with three adjacent proline and alanine rich
regions in the C-terminal half of human p57, and one in mouse p57. Alignments
were made using the Wisconsin Package (Genetics Computer Group) PILEUP
program and visual inspection. Allen, J. B., Walberg, M. W., Edwards, M.
C., and Elledge, S. J. (1995). Finding prospective partners in the library:
the two-hybrid system and phage display find a match. Trends in Biochem.
20, 511-516. Back to Figure 3 place in
text
Return to Finley
Lab Papers Page
Return to Finley
Lab Home Page
|

![]()
![]()
