|
The following is a draft versions
of a chapter to appear in "The yeast two-hybrid system"
(ed. P.L. Bartel and S. Fields) Oxford University Press, Oxford,
England
Two-hybrid analysis of genetic regulatory networks
Russell L. Finley Jr.1 and Roger
Brent2
1 Center for Molecular Medicine
and Genetics
Wayne State University School
of Medicine
540 East Canfield Avenue
Detroit, Michigan 48201
Phone (313) 577-7845
Fax (313) 577-5218
E-mail rfinley(at)wayne.edu
2 Department of Genetics
Harvard Medical School
and Department of Molecular Biology
Massachusetts General Hospital
50 Blossom street
Boston, Massachusetts 02114
Phone (617) 726-5925
Fax (617) 726-6893
E-mail brent(at)opal.mgh.harvard.edu
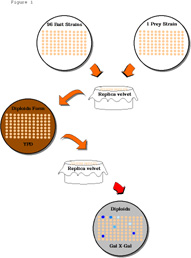
Table of contents
1. Introduction and Background
2. Interaction mating
2.1 Interaction mating - small scale
Protocol 1. Mating assay - small scale for tens of different
bait or prey proteins.
2.2 Interaction mating - large scale
Protocol 2. Collecting bait (and prey) strains
Protocol 3. Mating assay - large scale for hundreds of
different bait or prey strains.
3. Interaction mating assay with other
yeast two-hybrid systems
4. Recording the results
5. Interpreting interaction data
5.1 Qualitative interpretation
5.2 Quantitative interpretation
5.3 Inference of function from pattern
of interactions
6. Library scale and genome-wide characterization
of protein networks
7. Conclusions
8. Acknowledgments
9. References
1. Introduction and Background
There is a great need for general methods
to characterize the proteins that contemporary biology makes available.
The list of such proteins needing further characterization is
growing and includes proteins already known to be important for
specific cellular functions, mutant proteins identified in
vivo or made in vitro, and very large numbers of protein
being identified by genome projects. Here we describe the extension
of two-hybrid approaches so that they can bear on this problem.
The recent success of two-hybrid systems
is due to the fact that many cellular functions are carried out
by proteins that touch one another. For example, the complex process
of transcription initiation requires the ordered assembly of numerous
interacting transcription factors with RNA polymerase and ancillary
proteins, into a protein machine that initiates transcription
(Guarente, 1996; Tjian and Maniatis, 1994). This machine can be
viewed as a network of interacting proteins, as can the machines
that control other processes, such as DNA replication, protein
translation, and the cell cycle. A full understanding of these
processes will require knowledge of, not only the proteins (parts)
that make up each machine, but also of the topological relationships
(connections) that individual parts make with one another.
Likewise, a full understanding of the function
of any new protein will require knowledge of the interactions
it makes with previously identified proteins. Currently, most
new proteins are being identified by large scale sequencing projects.
For many of these new proteins the sequence alone sheds little
or no light on their function.
Two-hybrid systems have been used to probe
the function of new proteins ever since they were developed (Chien
et al., 1991; Fields and Song, 1989). The first application of
two-hybrid methods to probe protein function was to examine the
interactions between proteins isolated by two hybrid methods and
relatively small numbers of test proteins (see for example, Durfee
et al., 1993; Gyuris et al., 1993; Harper et al., 1993; Zervos
et al., 1993), but their use quickly spread to the analysis of
many other proteins (Choi et al., 1994; Kranz et al., 1994; Marcus
et al., 1994; Printen and Sprague, 1994; Van Aelst et al., 1993;
Yuan et al., 1993). In anticipation of the utility of applying
these methods to larger sets, we and others began devising ways
to do so.
Larger scale two hybrid approaches typically
rely on interaction mating. In this method the protein fused to
the DNA-binding domain (the bait) and the protein fused to the
activation domain (here called the prey) are expressed in two
different haploid yeast strains of opposite mating type (MATa
and MATa),
and the strains are mated to determine if the two proteins interact.
Mating occurs when haploid yeast strains of opposite mating type
come into contact, and results in fusion of the two haploids to
form a diploid yeast strain. Thus, an interaction can be determined
by measuring activation of a two-hybrid reporter gene in the diploid
strain.
As described below, interaction mating has
been used to examine interactions between small sets of tens of
proteins (Finley and Brent, 1994; Finley and Brent, 1995; Reymond
and Brent, 1995), larger sets of hundreds of proteins (R.L.F.
and R.B., unpublished), to screen libraries (Bendixen et al.,
1994), and to attempt to comprehensively map connections between
proteins encoded by a small genome (Bartel et al., 1996). The
primary advantage of this technique is that it reduces the number
of yeast transformations needed to test individual interactions.
For example, to test for interactions between a set of 10 bait
proteins and 5 prey proteins without interaction mating would
require 50 transformations to create 50 strains that carry the
pair-wise combinations of baits and preys. With mating however,
only 15 transformations would be needed; 10 for the different
bait plasmids, and 5 for the different prey plasmids; and the
resulting two sets of transformants would be mated to create the
50 combinations. The microbiology of the mating procedure (which
is extremely simple) is detailed in Section 2.
Interaction mating techniques have facilitated
a number of two-hybrid studies of protein protein interaction.
Among its first uses was to determine the specificity of interactors
isolated in library screens or interactor hunts (Harper et al.,
1993). As described in the previous chapters, in the first steps
of an interactor hunt, one isolates genes that encode proteins
that interact with a particular bait. Before the interacting proteins
are further characterized, it is necessary to determine if their
interaction with the bait is specific by showing that they do
not interact with other unrelated baits or with the DNA-binding
domain portion of the bait. When mating is used to test specificity,
the strain that contains the activation domain fused protein (prey)
is mated with different yeast strains which express either the
original bait protein or other, preferably unrelated baits, and
the investigator verifies that the reporters are only active in
diploids that contain the original bait (Finley and Brent, 1994;
Finley and Brent, 1995; Harper et al., 1993).
For example, Harper, Elledge and colleagues
used a mating assay to test the specificity of newly isolated
interactors (Harper et al., 1993). The methods of these investigators
also circumvented the need to isolate the prey plasmid. In their
experiments, they performed two-hybrid hunts with a bait plasmid
that contains a dominant marker, CYH2, that can be selected
against by plating the yeast on medium containing cycloheximide,
which is toxic to yeast that carry CYH2. Yeast isolated
in an interactor hunt were plated on cycloheximide plates to select
those that had lost the original bait plasmid but retained the
library plasmid. The resulting strain was then mated with a collection
of bait strains, including ones that expressed the original bait,
to determine the specificity of the library-encoded prey. A mating
scheme has also been used directly in an interactor hunt by mating
a strain expressing a bait with a strain transformed with the
library DNA; here, mating promises to bypass the need to perform
separate transformations with library DNA for each new hunt (Bendixen
et al., 1994).
In addition to its use in interactor hunts,
mating can be used to characterize small sets of proteins as described
in Section 2.1 and Protocol 1. In one example of this approach,
we used interaction mating to characterize a set of seven Drosophila
Cyclin-dependent kinases (Cdk) interactors, or Cdis (Finley and
Brent, 1994). Strains expressing versions of the Cdis fused to
an activation domain were mated with 74 different strains expressing
different bait proteins, including Cdks from other species and
four of the Cdis themselves. The results from this study illustrate
the types of information that can be derived from such a characterization.
First, the experiments showed that some of the Cdis interacted
with different subgroups of seven highly related Cdk baits, suggesting
that the Cdis recognize structural features shared by these Cdks
but absent in the non-interacting Cdks; inspection of an alignment
of the Cdk protein sequences suggested residues that may be important
for specific interactions with certain Cdis. Second, Cdi3, Drosophila
Cyclin D, interacted much more strongly with human Cdk4 than with
any of the other Cdks in the panel including the Drosophila
Cdks, suggesting that there may be an as yet unidentified Drosophila
Cdk4 homolog which is the true partner for Cyclin D. Third, two
of the Cdis interacted with two other Cdis, indicating in each
instance that each Cdi has surfaces for binding to the Cdk and
to another Cdi, and suggesting that these proteins form ternary
or higher order complexes. Finally, the demonstration that two
Cdis with no sequence similarity to previously identified proteins
interact with each other as well as with the Cdk, but not with
a panel of over 60 other proteins, provided an additional clue
to their functions, strongly supporting the idea that they function
along with the Cdk in the network of proteins that regulates the
cell cycle. These results demonstrate that examination of the
interactions between even small numbers of proteins can provide
a number of functional insights. Much larger sets of proteins
can be characterized by scaling up these procedures as described
in Section 2.2 and discussed in Sections 6 and 7.
2. Interaction mating
In this section we present methods for performing
interaction mating assays on small or large sets of proteins using
the interaction trap, and in Section 3 we discuss use of interaction
mating with other two-hybrid systems. The interaction trap (see
Chapter 4 and references therein) uses the E.coli protein
LexA as the DNA-binding domain and a protein encoded by random
E. coli sequences, the B42 "acid blob", as the
transcription activation domain. Both proteins are expressed from
multicopy (2µ) plasmids; the LexA fusion, or bait, is expressed
from a plasmid containing the HIS3 marker, and the activation
domain fused protein, or prey, is expressed from a plasmid containing
the TRP1 marker. In the most commonly used bait plasmid,
pEG202, the bait is expressed from the constitutive yeast ADH1
promoter. Related bait plasmids are available which express the
bait fused to a nuclear localization signal (pNLex, see Chapter
4), or which express the bait conditionally from the GAL1
promoter (pGILDA, D. Shaywitz and C. Kaiser, personal communication).
The most commonly used prey plasmid, pJG4-5, expresses proteins
fused to the B42 activation domain, the SV40 nuclear localization
signal, and an epitope tag derived from hemagglutinin, all driven
by the yeast GAL1 promoter which is active only in yeast
grown on galactose (Gyuris et al., 1993). Use of the GAL1
promoter to express the prey allows toxic proteins to be expressed
transiently and helps eliminate many false positives in interactor
hunts (Chapter 4). The interaction trap uses two reporter genes
that carry upstream LexA binding sites (operators): LEU2
and lacZ. The LEU2 reporters are integrated into
the yeast genome and the lacZ reporters typically reside
on 2µ plasmids bearing the URA3 marker, though integrated
versions are also available (R.L.F., R.B., S. Hanes, unpublished).
Several versions of the LEU2 and lacZ reporters
have been made that have a range of sensitivities based on the
number of upstream LexA operators. In general the LEU2
reporters are more sensitive to a given interacting pair of proteins
than the lacZ reporters (Estojak et al., 1995); however,
recently highly sensitive lacZ reporters have been used
that contain several LexA operators and transcription terminator
sequences downstream of the lacZ gene (S. Hanes, personal
communication).
Several different combinations of strains,
plasmids, and reporters can be used for mating (Section 3). In
one common version (Finley and Brent, 1994), the strain expressing
the bait (bait strain) is RFY206 (MATa ura3-52 his3Æ200
leu2-3 lys2Æ201 trp1::hisG) transformed with the HIS3
bait plasmid and a URA3 lacZ reporter plasmid like
pSH18-34. The strain expressing the activation domain-tagged protein
(prey strain) is EGY48 (MATa
ura3 his3 leu2::3LexAop-LEU2 trp1 LYS2) transformed with
the TRP1 prey plasmid. Patches of these two strains on
agar plates are brought into contact by replica plating (see below)
and grown on a rich medium overnight. During this time cells in
the patches mate and fuse to form diploids. The cells are then
transferred by replica plating to plates on which only diploids
can grow: these plates lack uracil, histidine, and tryptophan
so that neither parental haploid can grow on them. To avoid an
additional step, the diploid selection plates are also indicator
plates, which allows an interaction to be scored by testing for
expression of the reporter genes. In the protocols presented here
the lacZ reporter is measured, using diploid selection
indicator plates containing X-Gal, a chromogenic substrate for
the lacZ gene product. However, it is worth mentioning
that expression of the LEU2 reporter can also be easily
scored by putting the diploids on plates that lack leucine, and
that the future will likely bring other reporters. Furthermore,
because both reporter genes exhibit a reduced sensitivity in diploid
strains compared to haploid strains, the most sensitive versions
of the lacZ or LEU2 reporters are recommended for
interaction mating assays.
Variants of this simple procedure are sometimes
useful. In particular, because some baits activate transcription
by themselves, it is often useful to conditionally express the
prey protein so that one scores patches that show an increase
in reporter gene expression in the presence of the prey. To do
this, the diploids are placed on two different X-Gal plates, one
that contains galactose, which results in expression of the prey,
and one that contains glucose which represses expression of the
prey. Here, an interaction between the bait and prey is detected
when the diploid yeast containing them turn more blue on the galactose
X-Gal plate than on the glucose X-Gal plate.
2.1 Interaction mating - small scale
It is often informative to look for interactions
between small sets of proteins, or between a given protein and
a test set of ten to a hundred proteins. The test set, for example,
might contain different allelic forms of the original bait, sets
of structurally related proteins, sets of proteins known or suspected
to be involved in some process, and unrelated proteins used to
demonstrate the specificity of an interaction. Protocol 1 describes
a convenient method to test small sets of proteins for interactions.
The collections of bait and prey strains
used here can be maintained on yeast plates stored at 4oC
for two to three months, or stored frozen for several years (see
Protocol 2). For mating, the two strains are first streaked to
the appropriate selection plates: the bait strains (RFY206 containing
the URA3 lacZ reporter plasmid and HIS3 bait
plasmid) are streaked to plates lacking uracil and histidine -u-h
Glu) to maintain selection for the two plasmids; the prey strains
(EGY48 containing the TRP1 prey plasmid) are streaked to
plates lacking tryptophan (-w Glu) to maintain selection for the
prey plasmid. The haploid strains are then brought into contact
by placing both plates sequentially on the same replica velvet
and lifting the double imprint with a YPD plate (see Protocol
1). If the bait strains are streaked in parallel horizontal stripes
and the prey strains are streaked in vertical stripes, physical
contact between the strains will occur at the intersections of
the stripes on the YPD plate. After a brief period of growth to
allow diploids to form, the yeast are transferred to diploid selection
indicator plates by replica plating. Diploid colonies that contain
a pair of interacting bait and prey proteins are more blue on
the galactose X-Gal plate than the glucose X-Gal plate.
________________________________________________________________________
Protocol 1. Mating assay - small scale for tens of different
bait or prey proteins.
Materials
_ Bait strains: S. cerevisiae strain
RFY206 (MATa ura3-52 his3Æ200 leu2-3 lys2Æ201 trp1::hisG)
transformed with a URA3 plasmid containing a lacZ
reporter, such as pSH18-34, and various HIS3 bait plasmids,
such as derivatives of pEG202 that produce different LexA fusions.
Each bait strain will contain a different bait plasmid.
- Prey strains: S. cerevisiae strain EGY48 (MATa
ura3 his3 leu2::3LexAop-LEU2 trp1
LYS2) transformed with TRP1 prey plasmids, such as derivatives
of pJG4-5 that produce different activation domain-tagged proteins
or preys
- Sterile wooden applicator sticks (e.g. FisherBrand 01-340)
- Minimal glucose yeast plates lacking uracil and histidine
(-u-h Glu), see Chapter 4
- Minimal glucose plates lacking tryptophan (-w Glu), see Chapter
4
- YPD plates, see Chapter 4
- Minimal X-Gal glucose plates lacking uracil, histidine, and
tryptophan (-u-h-w Glu X-Gal), see Chapter 4.
- Minimal X-Gal galactose/raffinose plates lacking uracil, histidine,
and tryptophan (-u-h-w Gal/Raf X-Gal) , see Chapter 4
- Replica plater and sterile replica velvets
Optional
- Minimal glucose plates lacking uracil, histidine, tryptophan,
and leucine (-u-h-w-l Glu), see Chapter 4
- Minimal galactose/raffinose plates lacking uracil, histidine,
tryptophan, and leucine (-u-h-w- Gal/Raf), see Chapter 4
Method
1. Streak different bait strains in horizontal parallel stripes
on a -u-h Glu plate. Streaks should be at least 3 mm wide and
at least 5 mm apart, with the first streak starting about 15 mm
from the edge of the plate. A 100 mm plate (which for some reason
is typically 90 mm in diameter) will hold 8 different bait strains.
Create a duplicate plate of bait strains for each different plate
of prey strains to be used.
2. Likewise, streak different prey strains in vertical parallel
stripes on a -w Glu plate. As a control for baits that may activate
transcription, include a prey strain that contains the prey vector
pJG4-5 not encoding a fusion protein (i.e. encoding only the activation
domain). Create a duplicate plate of prey strains for each plate
of bait strains to be used.
3. Incubate plates at 30oC until there is
heavy growth on the streaks. When taken from reasonably fresh
cultures, for example plates that have been stored at 4oC
for less than a month, streaked RFY206-derived bait strains take
about 48 hours to grow and EGY48-derived prey strains take about
24 hours.
4. Press a plate of prey strains to a replica velvet, evenly and
firmly so that yeast from all along each streak are left on the
velvet. This plate may be reused if necessary. Press a plate of
bait strains to the same replica velvet. This plate of bait strains
cannot be reused as it is now contaminated with prey strains.
5. Lift the impression of the bait and prey strains from the velvet
by pressing a YPD plate on it. Incubate the YPD plate for 24 hours
at 30oC.
6. Replica YPD plates to the following diploid selection, indicator
plates: -u-h-w Glu X-Gal, -u-h-w Gal/Raf, and (optional: -u-h-w-l
Glu, and -u-h-w-l Gal/Raf). The YPD plate should contain sufficient
growth to enable a single impression on the velvet to be lifted
by at least four indicator plates.
7. Patch control strains (see text) onto the indicator plates
and incubate at 30oC. Examine results daily.
Diploids will grow and blue color will develop within 2 days.
________________________________________________________________________
2.2 Interaction mating - large scale
With a few modifications, the procedure described
above can be used to test for interactions between a single prey
protein and hundreds of baits (Protocol 3, see Figure 1
below). Large panels of bait strains can
be collected and stored frozen indefinitely (Protocol 2) and then
screened against any number of preys. One such set of bait strains
contains over 700 different LexA fusion proteins from our own
work and from numerous other labs that use the interaction trap
(R.L.F., R.B., A. Reymond, unpublished). Screening a protein against
such a panel enables one to quickly test its ability to interact
with a large number of known proteins, most of which have been
characterized to some extent, and have been chosen for study because
of their known or suspected involvement in some biological process.
Thus, the finding of an interaction between a tested protein and
a member of the panel can often lead to immediate clues about
the biological function of both proteins (see Section 5). While
the number of proteins in the existing panel is far less than
the number of proteins in a good library, this approach does offer
the advantage of screening the test protein against a set of proteins
enriched for those of current interest to the biological community.
It is worth noting that these proteins come from many different
organisms in which they are expressed in different tissues and
at different developmental stages. Thus it becomes possible to
identify interacting partners that have not yet been isolated
from the same species, or that are not expressed in tissues from
which interaction libraries have been made.
For some proteins, this approach offers additional
advantages over screening a library using a traditional two-hybrid
scheme. Proteins that activate transcription when fused to LexA
or another DNA-binding domain can be difficult to use in conventional
interactor hunts. Though methods are available to reduce the sensitivity
of the reporter genes (Durfee et al., 1993; Estojak et al., 1995;
Chapter 2, 3, 4) it is not always possible to reduce the reporter
sensitivity below the threshold of activation for some baits.
Moreover, reduction in reporter sensitivity carries with it the
risk that the reporters will not detect weakly interacting proteins.
Furthermore, spontaneously occurring yeast mutations, for example
those that increase the copy number of the bait plasmid, can increase
the activating potential of weakly activating baits (R.L.F., R.B.,
A. Mendelsohn, unpublished data); such mutations are typically
scored as positive in the early stages of an interactor hunt,
and they are not readily detected in schemes where the specificity
test is performed by removing the bait plasmid from the strain
containing the prey and mating the strain with other bait strains.
Thus, an alternative for proteins that activate transcription
as baits, is to use them as preys to screen existing panels of
baits, or even libraries of baits. Interaction mating approaches
also have clear advantages for proteins that are somewhat toxic
to yeast; the prey vector allows conditional expression of toxic
proteins in the presence of a bait, and often the interaction
can be observed as the reporters are activated even if the cells
are inviable. An example of the use of interaction mating together
with a large panel of bait strains to characterize a protein that
both activates transcription and is toxic to yeast, Drosophila
Cyclin E (Finley, Zavitz, Thomas, Richardson, Zipursky, and Brent,
in prep), is discussed in Section 7.
Figure 1. Mating assay for interactions between
a prey and 96 baits

Figure 1.
Top. The plate on the left holds 96
different yeast strains in patches (or colonies) that each express
a different bait protein. The plate on the right holds 96 patches,
each of the same yeast strain (prey strain) that expresses a protein
fused to an activation domain (prey). The plate of bait strains
and the plate of prey strains are each pressed to the same replica
velvet and the impression is lifted with a plate containing YPD
medium. After one day of growth on the YPD plate, during which
time the two strains mate to form diploids, the YPD plate is pressed
to a new replica velvet and the impression is lifted with a plate
containing diploid selection medium and an indicator like X-Gal.
Blue patches (dark spots) on the X-Gal plate indicate that the
lacZ reporter is transcribed, suggesting that the prey interacts
with the bait at that location.
________________________________________________________________________
Protocol 2. Collecting bait (and prey) strains
Materials
- Freezing media: 1:1 solution of minimal glucose media lacking
appropriate amino acids (e.g. -u-h Glu for bait strains) : sterile
glycerol solution (65% (v/v) glycerol, 0.1 M MgSO4, 25 mM Tris-HCl
pH 7.4)
- 1.0 to 1.5 ml cryotubes
- Yeast strains freshly streaked to minimal glucose plates
- Sterile wooden applicator strips
Methods
1. Streak bait strains to -u-h Glu plates, or prey strains to
-w Glu plates, and incubate at 30oC for 24
to 48 hours. Yeast should be taken from the plates and frozen
no more than 4 days after being streaked.
2. With a sterile wooden applicator stick, grab a dollop of yeast
from the plates and inoculate 0.5 ml of freezing solution in a
cryotube. Vortex lightly. This solution should have an OD600
over 3.0.
3. Alternatively, inoculate 0.5 ml of -u-h Glu liquid media to
an OD600 less than 0.2, incubate at 30oC
with shaking until OD600 = 1.5 to 2.0 (log
phase), and add 0.25 ml of this culture to 0.25 ml of sterile
glycerol solution in a cryotube.
4. Freeze by placing cryotubes in -80oC freezer.
Most strains can be recovered after up to at least two years by
scraping the surface of the ice and streaking to minimal glucose
plates. Avoid allowing entire contents of cryotube to thaw.
________________________________________________________________________
________________________________________________________________________
Protocol 3. Mating assay - large scale for hundreds of
different bait or prey strains.
Materials
- Freshly streaked bait and prey strains (see Protocol 1)
- One set of the following 150 x 15 mm plates for each test
of interactions between an activation domain-tagged protein (in
a prey strain) and 96 baits (bait strains): -u-h- Glu; -w Glu;
YPD; -u-h-w Glu X-Gal; -u-h-w Gal/Raf X-Gal
- Replica plater and sterile velvets for 150 mm diameter plates.
(A replica devise can be fashioned from a box of 200 µl pipet
tips by stretching a velvet over the top of the box)
- 96-prong device (e.g. DanKar MC-96) with 3 mm diameter flat
ended metal prongs in a 96-well configuration. Similar devices
can be used in 48-well configurations for use with 100 mm plates.
- 0.5 to 4.0 ml sterilized tubes arranged in a 96-well configurations
(e.g. cluster tubes such as Costar #4411). Ideally these tube
can be capped and frozen at -80oC.
- -u-h Glu liquid media, see Chapter 4
- -w Glu liquid media, see Chapter 4
- Sterile glycerol solution (65% (v/v) glycerol, 0.1 M MgSO4,
25 mM Tris-HCl pH 7.4)
Methods
1. It is most convenient to place large numbers of bait strains
in a 96-well configuration (Figure 1).
This can be done by inoculating 2 ml of -u-h Glu media in cluster
tubes and growing to OD600 = 1.5 to 2.0. After
making plates from these cultures (see step 2 below) add an equal
volume of sterile glycerol solution, cap and freeze at -80oC.
2. Use the 96-prong device, sterilized in ethanol and flame, to
transfer bait strains from the culture to the center of a 150
mm -u-h Glu plate. Each plate can contain 96 different bait strains.
Tens of identical plates can be made from one culture. Incubate
the plates at 30oC for 48 hours or until all
bait strains have grown to colonies 5 mm in diameter. These plates
can be stored at 4oC for up to 2 months and
used to inoculate another liquid culture when more plates are
needed. Several positions on each plate should contain control
strains with baits that activate various levels of transcription
(see Section 4 and Table 1).
3. Inoculate 50 ml of -w Glu liquid media with a prey strain and
grow at 30oC with shaking to OD600
= 1.5 to 3.0. Pour the culture into a sterile 150 mm plate, or
into the sterile top from a box of 200 µl pipets, and use
the 96-prong device, sterilized in ethanol and flame, to transfer
the culture to -w Glu plates. On these plates, all 96 positions
will contain the same prey strain.
4. Follow the replica plating procedure from Protocol 1 to combine
the bait and prey strains to a YPD plate, and then after growth
on the YPD plate at 30oC for 24 hours, replica
to X-Gal indicator, diploid selection plates (-u-h-w Glu X-Gal
and -u-h-w Gal/Raf X-Gal) (see Figure 1 above).
5. Examine results after two days.
________________________________________________________________________
3. Interaction mating assay with other
yeast two-hybrid systems
In addition to the interaction trap, many
other yeast two-hybrid systems have been developed (see Chapter
1 and Allen et al., 1995; Fields and Sternglanz, 1994; Mendelsohn
and Brent, 1994, for reviews). All of these allow the analysis
of individual protein-protein interactions, and permit interactor
hunts to isolate new proteins that interact with a bait. In some
instances plasmids or strains from one system can be used in another,
but often the components are incompatible. Most often, the yeast
selectable markers on the different components differ. In addition,
systems that use Gal4 as the DNA binding domain cannot be used
with yeast strains that have a wild-type GAL4 gene, and
therefore, since the Gal4 protein is required to activate the
GAL1 promoter, cannot be used with systems that use the
GAL1 promoter to drive expression of the prey protein.
Finally, use of interaction mating requires careful attention
to the mating types of the strains and the selectable markers
used to select the diploids.
4. Recording the results
Interaction between bait and prey results
in the interaction phenotypes: growth of the strain on
medium lacking leucine, and transcriptional activation of the
lacZ reporter and production of active ß-galactosidase.
On X-Gal plates the ß-galactosidase cleaves the X-Gal substrate,
producing a product which turns the yeast colony blue. The amount
of color provides a fast and simple method to approximate the
level of lacZ expression in a strain. An interaction is
scored when a the diploid colony is more blue on the X-Gal plate
containing galactose than the X-Gal plate containing glucose.
Scoring these interactions benefits from
inclusion of a number of controls. To control for common variations
between the X-Gal plates, it is useful to include control strains
that contain baits which activate transcription to varying extents.
Table 1 shows some baits with known
activating abilities. Inclusion of such strains on every X-Gal
plate enables one to normalize the amount of blue produced by
an interaction. It is also useful to include a control strain
to check that the plates contain the correct carbon sources, and
ensure that the GAL1 promoter which drives the expression
of the prey protein is activated on the Gal/Raf plates and not
the Glu plates. An ideal control of this nature consists of a
diploid strain derived from a mating assay, which expresses an
interacting pair of bait and prey proteins, such as any one of
a number of well-characterized interacting pairs (Finley and Brent,
1994; Gyuris et al., 1993; Zervos et al., 1993). An alternative
to using X-Gal plates is to perform a filter lift assay for ß-galactosidase
activity in grown diploid colonies (Chapter ). Finally, every
bait should be tested to see if, and how much, it activates transcription
in the absence of a prey, which can be simply accomplished by
mating the bait strains to a strain containing the empty prey
vector. Thus, a true interaction with a prey protein is scored
when the amount of galactose-dependent activation of the lacZ
reporter (e.g. amount of blue) exceeds the amount produced in
the absence of a prey.
Table 1. Activating
and non-activating baits
5. Interpreting interaction data
5.1 Qualitative interpretation
For large amounts of information flowing
from interaction mating experiments, the problem of determining
whether individual interactions are meaningful is multiplied.
We consider a number of these separately.
True and false positives.
Any given interaction with affinity tighter than 10-6
will get detected. Although there may exist a weak positive correlation
between apparent tightness and biological significance, many apparently
weak interactions are real while some strong ones are not. The
problem of determining which interactions have biological significance
is therefore not trivial. At the moment, the most satisfying way
to show biological significance is to verify the interaction by
a different, biochemical technique, preferably co-precipitation
from a cell in which both proteins are expressed. However, the
interaction data alone can often point out probable true and false
positives. For example, our experience indicates that highly specific
interactions, such as between a protein that binds to one or a
small set of highly related proteins and not to hundreds of unrelated
proteins, are good candidates to pursue as biologically relevant.
Conversely, we tend to give less weight to interactions between
proteins that are sticky, or involving those proteins so ubiquitous
in the life of the cell (e.g., members of the ubiquitin system
or heat shock proteins) that the interactions might be meaningful
but relatively uninformative.
True and false negatives.
A problem less frequently considered is that of interactions that
are not observed. Two observations suggest that many interactions
that should be observed are not. One is that in library screens
proteins that should be found occasionally are not. Although failure
to recover expected proteins in this instance might be due to
trivial considerations, such as the absence of the protein from
the library used, another fact suggests there could be other reasons.
There are now a number of examples in which known interactions
are either not observed, or are subject to directionality, being
observed only when one of the two proteins is a bait and the other
a prey (see for example, Estojak et al., 1995). Our current doctrine
for determining that individual interactions do not occur is that
full length and truncated putative partners must be tested in
all combinations of baits and preys, with the most sensitive reporters,
before the investigator can tentatively conclude that the two
proteins do not touch. Since this is impractical for mating experiments
that involve a large number of baits and preys, such as the genome
wide approaches discussed below, we are resigned that false negatives
will arise, and we do not give the absence of interaction any
weight in our data analysis. This doctrine may change as more
sensitive detection methods are designed.
Multimeric complexes.
Finally, it is worth noting that one can build up chains of individual
binary interactions to suggest higher order complexes. This has
worked well, for example with proteins in signal transduction
(Choi et al., 1994; Marcus et al., 1994; Printen and Sprague,
1994), and the advent of mating techniques has made it even easier
to build up such patterns (Finley and Brent, 1994; C. Kaiser and
D. Shaywitz, personal communication).
5.2 Quantitative interpretation
No two-hybrid technique - particularly the
mating techniques described in Protocols 1 and 3 - allows precise
quantitation, and any interactions identified must be studied
further to determine biological significance and biophysical characteristics.
However, some quantitative information does inhere in the data.
The amount of ß-galactosidase activity in the cell is proportional
to the level of lacZ transcription so that some information
about the strength of interaction of two proteins might be derived
from measuring ß-galactosidase activity. Though measurement
of ß-galactosidase activity with a liquid assay (Guarente
and Ptashne, 1981; Rose and Botstein, 1983) is not practical for
large numbers of strains, a less precise indication of enzyme
activity can be derived from the color of the yeast colony on
an X-Gal indicator plate; for example, dark blue, light blue,
or white colonies correspond to high, moderate, or low to no lacZ
transcription. Despite this correlation between transcription
levels and ß-galactosidase activity, one must use caution
in using ß-galactosidase activity to compare relative affinities
of different bait and prey pairs. Many variables could affect
the interaction phenotypes, including the stability of the two
fusion proteins, transport of the fusions into the nucleus, and
the ability of the bait to bind DNA. These considerations make
it imprudent to use two-hybrid data to compare affinities between
sets of unrelated proteins.
It is, however, often possible to make meaningful
comparisons of the affinity of a single prey protein for several
related baits. Such a comparison relies on two assumptions that
are generally correct and can be experimentally verified: that
the prey, which can be detected with antibodies to its epitope
tag, is expressed at the same level in each diploid, and that
the baits, which can be detected with anti-LexA antibody and whose
DNA binding can be quantitated by a repression assay (Brent and
Ptashne, 1984), occupy the operators to similar extents.
5.3 Inference of function from pattern
of interactions
One reason for developing interaction mating
techniques was the hope that it would reveal contacts between
test proteins and known proteins that would provide clues to the
function of the test proteins. This turned out be true (see for
example, Section 7). However, our first experiments revealed that
clues to function might also be derived from the pattern of interactions
a protein makes, without reference to the biochemical identity
of the interacting proteins. A simple example, taken from our
first experiments, illustrates this point. Cdi4 and Cdi11 both
interact with Drosophila Cdc2c and interaction mating experiments
also revealed that Cdi4 interacts with Cdi11 (Finley and Brent,
1994). From the pattern of interactions alone, these data are
consistent with the idea that Cdi4, Cdi11 and Cdc2c could form
a three protein complex. It is possible that other such patterns
of interactions, particularly conjoined with the crude affinity
data, might signal other sorts of regulators. The algorithmic
analysis of connectivity data for patterns of this type is an
important area of future research.
6. Library scale and genome-wide characterization
of protein networks
Interaction mating schemes can also be used
on a larger scale, for screening libraries, and, eventually to
characterize complex genomes. One such scheme is to mate a pool
of cells containing different activation domain-tagged proteins
against a bait protein. Another is the converse of the original
two-hybrid system. In this approach, a library of different proteins
fused to a DNA-binding domain is used in an interactor hunt to
find proteins that interact with a specific activation-tagged
protein. Historically, the drawback to such approaches has been
that libraries that express proteins fused to DNA-binding domains
will contain a large number proteins that activate transcription
when brought to DNA (Ma and Ptashne, 1987), complicating the task
of identifying yeast in which the reporters are active due to
the presence of an interacting protein. One way to circumvent
this difficulty would be to introduce the library into a yeast
strain that contained a counter-selectable reporter gene (e.g.
LexAop-LYS2 and LexAop-URA3), select against those yeast
that contained activators, and then mate the "depleted"
library with yeast of the opposite mating type that contain the
test protein. Yet another way is to express the activation domain-tagged
proteins from a conditional promoter like GAL1 and compare
reporter activation between replica plates on which they are and
are not expressed, as descried in Protocol 1 and 3, and in Chapter
4).
Recently, Bartel et al applied two-hybrid
technology to characterize a small genome (Bartel et al., 1996).
They set out to identify all detectable binary interactions between
proteins encoded by the bacteriophage T7 genome. They did this
by making two libraries, one of DNA-binding domain hybrids and
one of activation domain hybrids, expressed in yeast strains of
opposite mating type. They then mated a pool of yeast that contained
the entire library of activation domain hybrids with 30,000 of
the strains expressing DNA-binding domain fusions, in groups of
ten so they could readily single out those that activated transcription.
They selected diploids in which the HIS3 reporter was activated
and screened for activation of a second lacZ reporter using
a filter assay. In this way they identified 19 binary interactions
between T7 encoded proteins. They further performed individual
interactor hunts testing 34 specific DNA-binding hybrids against
the entire activation domain library, and 11 specific activation
domain hybrids against the entire DNA-binding domain hybrid library,
again by interaction mating, and identified 3 additional interactions.
Finally, they made a matrix of all of the yeast expressing DNA-binding
domain hybrids involved in an interaction mated with yeast expressing
all of the activation domain hybrids involved in an interaction
to identify three more interactions.
By this means they detected a total of 25
interactions. Some of the interactions were previously known,
while others confirmed interactions that had been suspected based
on genetic or biochemical studies. Most importantly, 10 of the
interactions detected in this two-hybrid tour de force identified
connections between proteins not previously known to interact.
This new information contains both clues to the function of individual
proteins and clues as to how some may function together. An additional
windfall from this approach, made possible by the fact that the
two libraries were made from random fragments of the T7 genome,
was the identification of a number of previously unsuspected intramolecular
interactions. The detection of these intramolecular interactions
suggested possible homo-oligomeric protein contacts as well as
interdomain contacts that might promote the formation of tertiary
structure. The success of this genome-wide approach demonstrates
that interaction mating techniques can be used to identify the
networks of interacting proteins encoded by more complex genomes.
The charting of such connections between proteins will provide
insights into the functions of individual proteins and lead to
a better understanding of how groups of proteins control biological
processes.
7. Conclusions
The few years since the advent of two-hybrid
systems has proven their utility in the study of defined protein
interactions, in identification of new interacting proteins, and
in the charting of genetic networks of proteins involved in processes
from signal transduction to transcription regulation. These tremendous
successes suggest that two-hybrid approaches like those discussed
in this chapter may eventually be used to identify all of the
protein protein contacts made in a cell or an organism.
Before this time, another need is clear.
Sequencing projects like the human genome initiative will soon
provide us with the sequences of all of the expressed proteins.
A good deal of insight into the function of these proteins can
be derived from their sequences alone, but ultimately must be
combined with other forms of information to understand the biology
in detail. Information about contacts made by the proteins of
a genome will complement and augment the sequence information.
Such information will likely come from incremental scaling up
of the methods described here, as well as from scaled up versions
of ideas such as those developed by Bartel et al (Bartel et al.,
1996). Connection data will also come from the thousands of labs
using two-hybrid systems to identify and characterize specific
proteins. Finally, it may also come from recent efforts to identify
all of the proteins in the networks of interacting proteins in
a cell using rapid sequential two-hybrid interactor hunts that
use the proteins isolated in one hunt as starting points for further
hunts, in a sort of "protein interaction walk" (R.L.F.,
unpublished).
As discussed in Section 5, all two-hybrid
approaches inevitably produce false positives, interactions that
do not occur in any biological setting. Thus, although it will
be rich in information, connectivity maps derived from two-hybrid
data will necessarily be imprecise. This need not be thought of
as a significant drawback of genome-wide two-hybrid approaches,
provided it is borne in mind that the information in a protein
linkage map derives its utility in providing clues to important
interactions which must be explored with further study using other
methods.
One example of an insight into protein function
from a large scale two-hybrid approach is the identification of
the Drosophila protein Roughex, Rux, as a protein that
interacts strongly and specifically with Drosophila Cyclin
E (Finley, Zavitz, Thomas, Richardson, Zipursky, and Brent, in
prep). Rux, a 335 amino acid protein whose sequence gives no clues
to its function (Thomas et al., 1994), was in a panel of 600 bait
proteins that we tested for interaction with a Cyclin E prey.
It was known that rux is required for normal eye development;
loss of function rux mutants have rough eyes and aberrant
cell cycle regulation in the eye imaginal disc from which the
eye develops (Thomas et al., 1994). Thomas et al showed that a
stripe of cells in the morphogenetic furrow of the developing
eye disc must arrest transiently in the G1 phase of the cell cycle
for proper development and this G1 arrest fails in rux mutant
eye discs. Combined with this information, the finding that Rux
interacts directly with Cyclin E, a protein known to be required
for progression through G1, immediately suggested that Rux modulated
cyclin activity, and inspired us to undertake specific genetic
and biochemical experiments to test the hypothesis.
Scaled up interaction mating assays are likely
to be useful in the analysis of genetic diseases and other complex
genetic traits. The first version of this idea, which has a long
history, is that genes that modify the function of other genes
may participate in the same process. A less obvious corollary
of this idea became apparent several years ago: that, among the
proteins that interact with a protein involved in a disease, those
that interact differently with wild-type and disease state allelic
forms of the protein are likely to be involved in the disease.
Recently, Reymond and Brent undertook a test of this idea (Reymond
and Brent, 1995). They studied the protein encoded by the INK4
human tumor suppressor gene, p16. Wild type p16 interacts with
two human Cyclin-dependent kinases, Cdk4 and Cdk6 to inhibit their
activity. As expected, interaction mating showed that alleles
of p16 found in cancer-prone families are deficient in their interaction
with the kinases. Two unexpected conclusions arose from these
experiments. One allele, p16-G101W, showed decreased interaction
with Cdk4 but not with Cdk6, suggesting that its role in disease
is unrelated to its action on Cdk6. Furthermore, another allele,
p16-I49T, which is also found in the control population, is deficient
in interaction with Cdk4, suggesting that this allele may also
contribute to a tumor-prone phenotype. These findings underscore
the fact that interaction mating with different alleles in a population
will contribute to the analysis of complex polygenic traits.
The ability to conduct scaled-up two hybrid
analysis has come at a good time. The trickle of new genes and
alleles has become a torrent. Robust and general approaches to
the understanding of gene and pathway function will help us to
the next step of biological understanding.
Back to Finley Lab Home Page
7. Acknowledgments
We thank L. Lok and members of the Brent
laboratory, past and present, for helpful discussions, A. Mendelsohn
for assistance in working out the interaction mating assay, and
A. Reymond for help in collecting and maintaining the bait panel.
We also thank P. Colas, E. Golemis, and C. Giroux for helpful
comments on the manuscript. R.B. was supported by Hoescht AG and
an American Cancer Society Faculty Research Award.
8. References
Allen, J. B., Walberg, M. W., Edwards, M.
C., and Elledge, S. J. (1995). Finding prospective partners in
the library: the two-hybrid system and phage display find a match.
Trends in Biochem. 20, 511-516.
Bartel, P. L., Roecklein, J. A., SenGupta,
D., and Fields, S. (1996). A protein linkage map of Escherichia
coli bacteriophage T7. Nature Genetics 12, 72-77.
Bendixen, C., Gangloff, S., and Rothstein,
R. (1994). A yeast mating-selection scheme for detection of protein-protein
interactions. Nucleic Acids Research 22, 1778-1779.
Brent, R., and Ptashne, M. (1984). A bacterial
repressor protein or a yeast transcriptional terminator can block
upstream activation of a yeast gene. Nature 312, 612-615.
Chien, C.-T., Bartel, P. L., Sternglanz,
R., and Fields, S. (1991). The two-hybrid system: A method to
identify and clone genes for proteins that interact with a protein
of interest. Proc. Natl. Acad. Sci. USA 88, 9578-9582.
Choi, K. Y., Satterberg, B., Lyons, D. M.,
and Elion, E. A. (1994). Ste5 tethers multiple protein kinases
in the MAP kinase cascade required for mating in S. cerevisiae.
Cell 78, 499-512.
Durfee, T., Becherer, K., Chen, P.-L., Yeh,
S.-H., Yang, Y., Kilburn, A. E., Lee, W.-H., and Elledge, S. J.
(1993). The retinoblastoma protein associates with the protein
phophatase type 1 catalytic subunit. Genes and Dev. 7,
555-569.
Estojak, J., Brent, R., and Golemis, E. A.
(1995). Correlation of two-hybrid affinity data with in vitro
measurements. Mol Cell Biol 15, 5820-5829.
Fields, S., and Song, O. (1989). A novel
genetic system to detect protein-protein interactions. Nature
340, 245-246.
Fields, S., and Sternglanz, R. (1994). The
two-hybrid system: an assay for protein-protein interactions.
Trends Genet. 10, 286-292.
Finley, R. L., Jr., and Brent, R. (1994).
Interaction mating reveals binary and ternary connections between
Drosophila cell cycle regulators. Proc Natl Acad Sci U S A 91,
12980-12984.
Finley, R. L., Jr., and Brent, R. (1995).
Interaction trap cloning with yeast. In DNA Cloning 2, Expression
Systems: A Practical Approach, B. D. Hames and D. M. Glover, eds.
(Oxford: Oxford University Press), pp. 169-203.
Golemis, E.A., and Brent, R. (1992). Fused
protein domains inhibit DNA binding by LexA. Mol. Cell. Biol.
12, 3006-3014.
Guarente, L. (1996). Transcriptional coactivators
in yeast and beyond. Trends in Biochem 20, 517-521.
Guarente, L., and Ptashne, M. (1981). Fusion
of Eschericia coli lacZ to the cytochrome c gene of Saccharomyces
cerevisiae. Proc. Natl. Acad. Sci. USA 78, 2199-2203.
Gyuris, J., Golemis, E., Chertkov, H., and
Brent, R. (1993). Cdi1, a human G1 and S phase protein phosphatase
that associates with Cdk2. Cell 75, 791-803.
Harper, J. W., Adami, G. R., Wei, N., Keyomarsi,
K., and Elledge, S. J. (1993). The p21 Cdk-interacting protein
Cip1 is a potent inhibitor of g1 cyclin-dependent kinases. Cell
75, 805-816.
Kranz, J. E., Satterberg, B., and Elion,
E. A. (1994). The MAP kinase Fus3 associates with and phosphorylates
the upstream signaling component Ste5. Genes Dev 8, 313-27.
Lech, K., Anderson, K., and Brent, R. (1988).
DNA-bound Fos proteins activate transcription in yeast. Cell 52,
179-184.
Ma, J., and Ptashne, M. (1987). A new class
of transcriptional activators. Cell 51, 113-119.
Marcus, S., Polverino, A., Barr, M., and
Wigler, M. (1994). Complexes between STE5 and components of the
pheromone-responsive mitogen-activated protein kinase module.
Proc. Natl. Acad. Sci. USA 91, 7762-7766.
Mendelsohn, A. R., and Brent, R. (1994).
Applications of interaction traps/two-hybrid systems to biotechnology
research. Curr. Op. Biotechn. 5, 482-486.
Printen, J. A., and Sprague, G. F., Jr. (1994).
Protein-protein interactions in the yeast pheromone response pathway:
Ste5p interacts with all members of the MAP kinase cascade. Genetics
138, 609-619.
Reymond, A., and Brent, R. (1995). p16 proteins
from melanoma-prone families are deficient in binding to Cdk4.
Oncogene 11, 1173-1178.
Rose, M., and Botstein, D. (1983). Construction
and use of gene fusions to lacZ (beta-galactosidase) that are
expressed in yeast. Methods Enzymol 101, 167-80.
Thomas, B. J., Gunning, D. A., Cho, J., and
Zipursky, L. (1994). Cell cycle progression in the developing
Drosophila eye: roughex encodes a novel protein required for the
establishment of G1. Cell 77, 1003-1014.
Tjian, R., and Maniatis, T. (1994). Transcriptional
activation: A complex puzzle with few easy pieces. Cell 77,
5-8.
Van Aelst, L., Barr, M., Marcus, S., Polverino,
A., and Wigler, M. (1993). Complex formation between RAS and RAF
and other protein kinases. Proc. Natl. Acad. Sci., U.S.A. 90,
6213-6217.
Yuan, Y. O., Stroke, I. L., and Fields, S.
(1993). Coupling of cell identity to signal response in yeast:
interaction between the alpha 1 and STE12 proteins. Genes Dev
7, 1584-97.
Zervos, A. S., Gyuris, J., and Brent, R.
(1993). Mxi1, a protein that specifically interacts with Max to
bind Myc-Max recognition sites. Cell 72, 223-232.
Back to Finley Lab Home Page
|

![]()
![]()
